Visualization
Welcome to the cooltools visualization notebook!
Visualization is a crucial part of analyzing large-scale datasets. Before performing analyses of new Hi-C datasets, it is highly recommend to visualize the data. This notebook contains tips and tricks for visualization of coolers using cooltools.
Current topics:
Smoothing, interpolation, and adaptive coarsegraining
Future topics:
higlass-python
translocations, structural variants
visualizing matrices for other organisms
[1]:
# import standard python libraries
import numpy as np
import matplotlib.pyplot as plt
import seaborn as sns
import pandas as pd
import os
[2]:
# download test data
# this file is 145 Mb, and may take a few seconds to download
import cooltools
data_dir = './data/'
cool_file = cooltools.download_data("HFF_MicroC", cache=True, data_dir=data_dir)
print(cool_file)
./data/test.mcool
[3]:
#import python package for working with cooler files: https://github.com/open2c/cooler
import cooler
Inspecting C data
The file we just downloaded, test.mcool, contains Micro-C data from HFF cells for two chromosomes in a multi-resolution mcool format.
[4]:
# to print which resolutions are stored in the mcool, use list_coolers
cooler.fileops.list_coolers(f'{data_dir}/test.mcool')
[4]:
['/resolutions/1000',
'/resolutions/10000',
'/resolutions/100000',
'/resolutions/1000000']
[5]:
### to load a cooler with a specific resolution use the following syntax:
clr = cooler.Cooler(f'{data_dir}/test.mcool::resolutions/1000000')
### to print chromosomes and binsize for this cooler
print(f'chromosomes: {clr.chromnames}, binsize: {clr.binsize}')
### to make a list of chromosome start/ends in bins:
chromstarts = []
for i in clr.chromnames:
print(f'{i} : {clr.extent(i)}')
chromstarts.append(clr.extent(i)[0])
chromosomes: ['chr2', 'chr17'], binsize: 1000000
chr2 : (0, 243)
chr17 : (243, 327)
Coolers store pairwise contact frequencies in sparse format, which can be fetched on demand as dense matrices. clr.matrix returns a matrix selector. The selector supports Python slice syntax [] and a .fetch() method. Slicing clr.matrix() with [:] fetches all bins in the cooler. Fetching can return either balanced, or corrected, contact frequences (balance=True), or raw counts prior to bias removal (balance=False).
In genome-wide C data for mammalian cells in interphase, the following features are typically observed:
Higher contact frequencies within a chromosome as opposed to between chromosomes; this is consistent with observations of chromosome territories. See below.
More frequent contacts between regions at shorter genomic separations. Characterizing this is explored in more detail in the contacts_vs_dist notebook.
A plaid pattern of interactions, termed compartments. Characterizing this is explored in more detail in the compartments notebook.
Each of these features are visible below.
Visualizing C data
Plotting raw counts
First, we plot raw counts with a linear colormap thresholded at 500 counts for the entire cooler. Note that the number of counts per cooler depends on the sequencing depth of the experiment, and a different threshold may be needed to see the same features.
[6]:
f, ax = plt.subplots(
figsize=(7,6))
im = ax.matshow((clr.matrix(balance=False)[:]),vmax=500);
plt.colorbar(im ,fraction=0.046, pad=0.04, label='raw counts')
ax.set(xticks=chromstarts, xticklabels=clr.chromnames,
xlabel='position, chrom#', ylabel='position, bin#')
ax.xaxis.set_label_position('top')
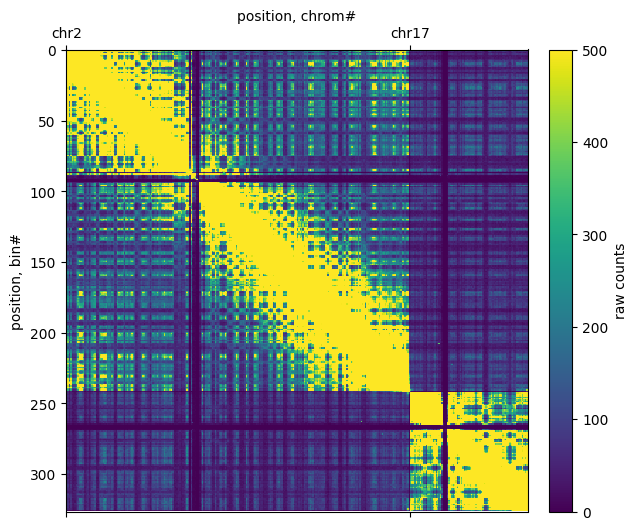
Plotting subregions
Below, we fetch and plot an individual chromosome (left) and a region of a chromosome (right) using clr.fetch()
[7]:
# to plot ticks in terms of megabases we use the EngFormatter
# https://matplotlib.org/gallery/api/engineering_formatter.html
from matplotlib.ticker import EngFormatter
bp_formatter = EngFormatter('b')
def format_ticks(ax, x=True, y=True, rotate=True):
if y:
ax.yaxis.set_major_formatter(bp_formatter)
if x:
ax.xaxis.set_major_formatter(bp_formatter)
ax.xaxis.tick_bottom()
if rotate:
ax.tick_params(axis='x',rotation=45)
f, axs = plt.subplots(
figsize=(14,4),
ncols=3)
ax = axs[0]
im = ax.matshow(clr.matrix(balance=False)[:], vmax=2500);
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
ax.set_xticks(chromstarts)
ax.set_xticklabels(clr.chromnames)
ax.set_yticks(chromstarts)
ax.set_yticklabels(clr.chromnames)
ax.xaxis.tick_bottom()
ax.set_title('All data')
ax = axs[1]
im = ax.matshow(
clr.matrix(balance=False).fetch('chr17'),
vmax=2500,
extent=(0,clr.chromsizes['chr17'], clr.chromsizes['chr17'], 0)
);
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
ax.set_title('chr17', y=1.08)
ax.set_ylabel('position, Mb')
format_ticks(ax)
ax = axs[2]
start, end = 30_000_000, 60_000_000
region = ('chr17', start, end)
im = ax.matshow(
clr.matrix(balance=False).fetch(region),
vmax=2500,
extent=(start, end, end, start)
);
ax.set_title(f'chr17:{start:,}-{end:,}', y=1.08)
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
format_ticks(ax)
plt.tight_layout()
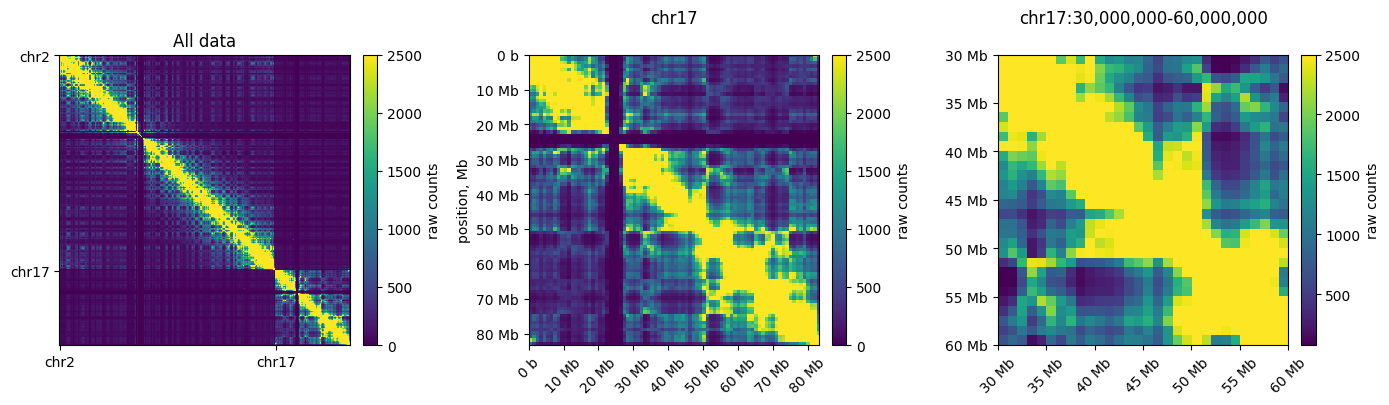
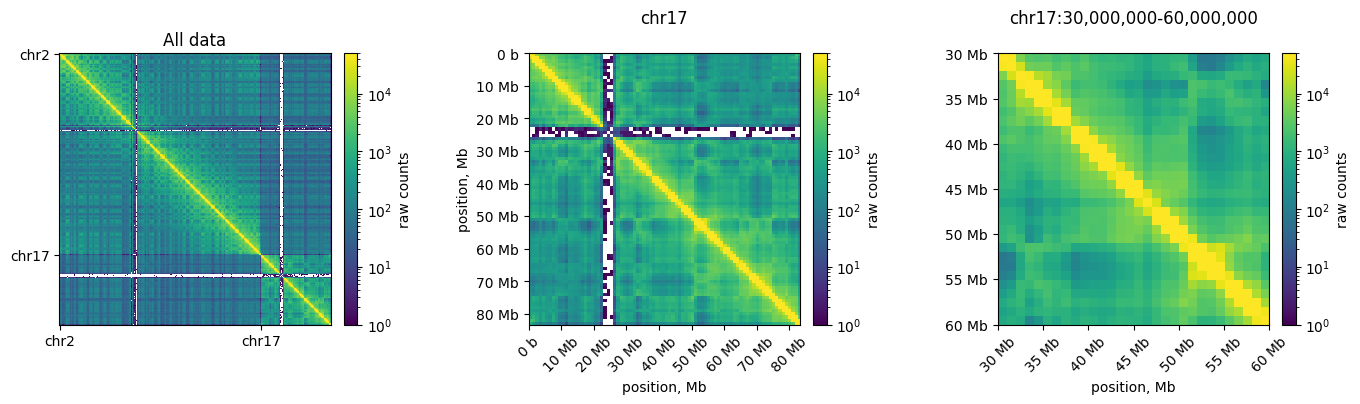
Logarithmic color scale
Since C data has a high dynamic range, we often plot the data in log-scale. This enables simultaneous visualization of features near and far from the diagonal in a consistent colorscale. Note that regions with no reported counts are evident as white stripes at both centromeres. This occurs because reads are not uniquely mapped to these highly-repetitive regions. These regions are masked before matrix balancing.
[8]:
# plot heatmaps at megabase resolution with 3 levels of zoom in log-scale with a consistent colormap#
from matplotlib.colors import LogNorm
f, axs = plt.subplots(
figsize=(14,4),
ncols=3)
bp_formatter = EngFormatter('b')
norm = LogNorm(vmax=50_000)
ax = axs[0]
im = ax.matshow(
clr.matrix(balance=False)[:],
norm=norm,
)
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
ax.set_xticks(chromstarts)
ax.set_xticklabels(clr.chromnames)
ax.set_yticks(chromstarts)
ax.set_yticklabels(clr.chromnames)
ax.xaxis.tick_bottom()
ax.set_title('All data')
ax = axs[1]
im = ax.matshow(
clr.matrix(balance=False).fetch('chr17'),
norm=norm,
extent=(0,clr.chromsizes['chr17'], clr.chromsizes['chr17'], 0)
);
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
ax.set_title('chr17', y=1.08)
ax.set(ylabel='position, Mb', xlabel='position, Mb')
format_ticks(ax)
ax = axs[2]
start, end = 30_000_000, 60_000_000
region = ('chr17', start, end)
im = ax.matshow(
clr.matrix(balance=False).fetch(region),
norm=norm,
extent=(start, end, end, start)
);
ax.set_title(f'chr17:{start:,}-{end:,}', y=1.08)
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='raw counts');
ax.set(xlabel='position, Mb')
format_ticks(ax)
plt.tight_layout()
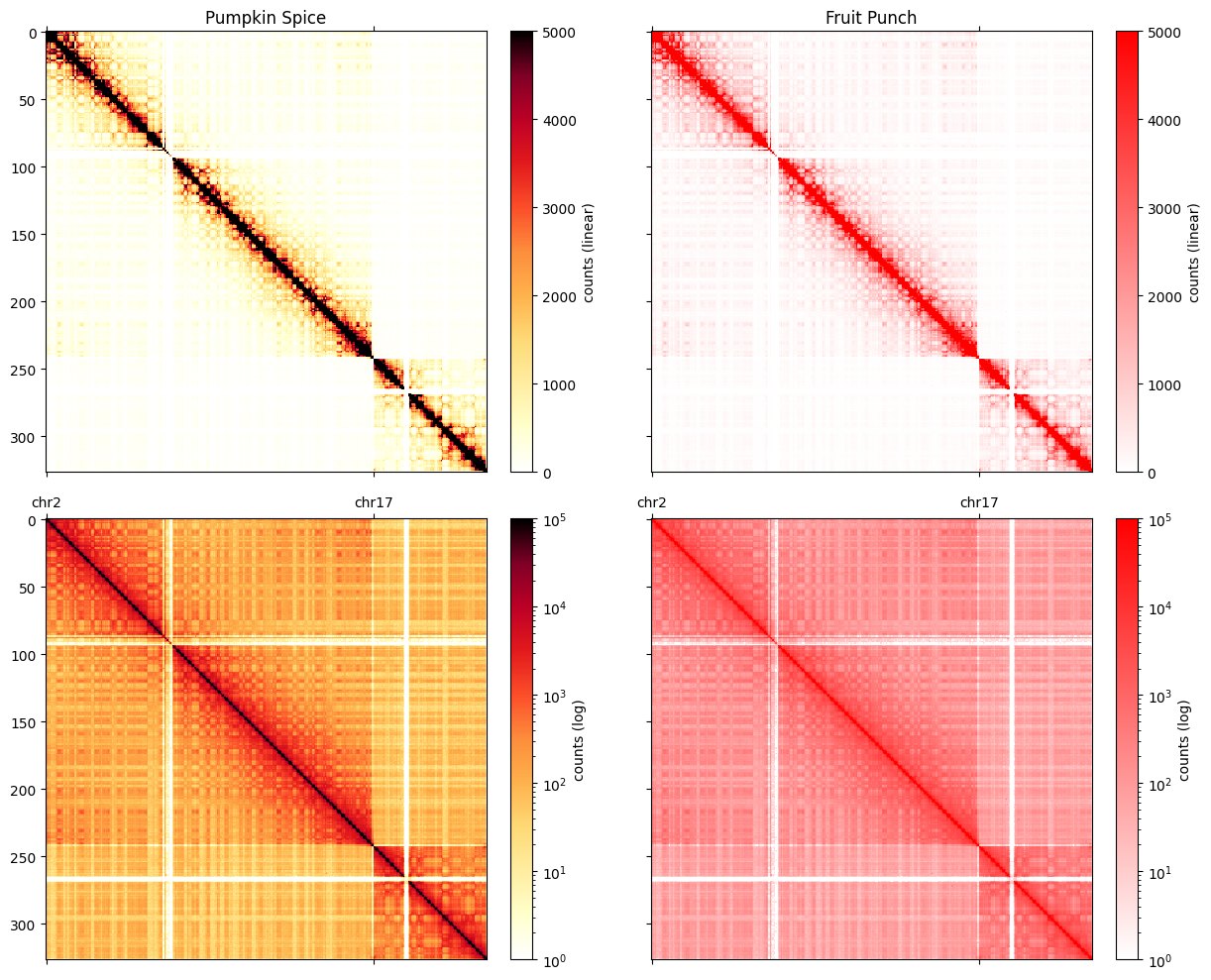
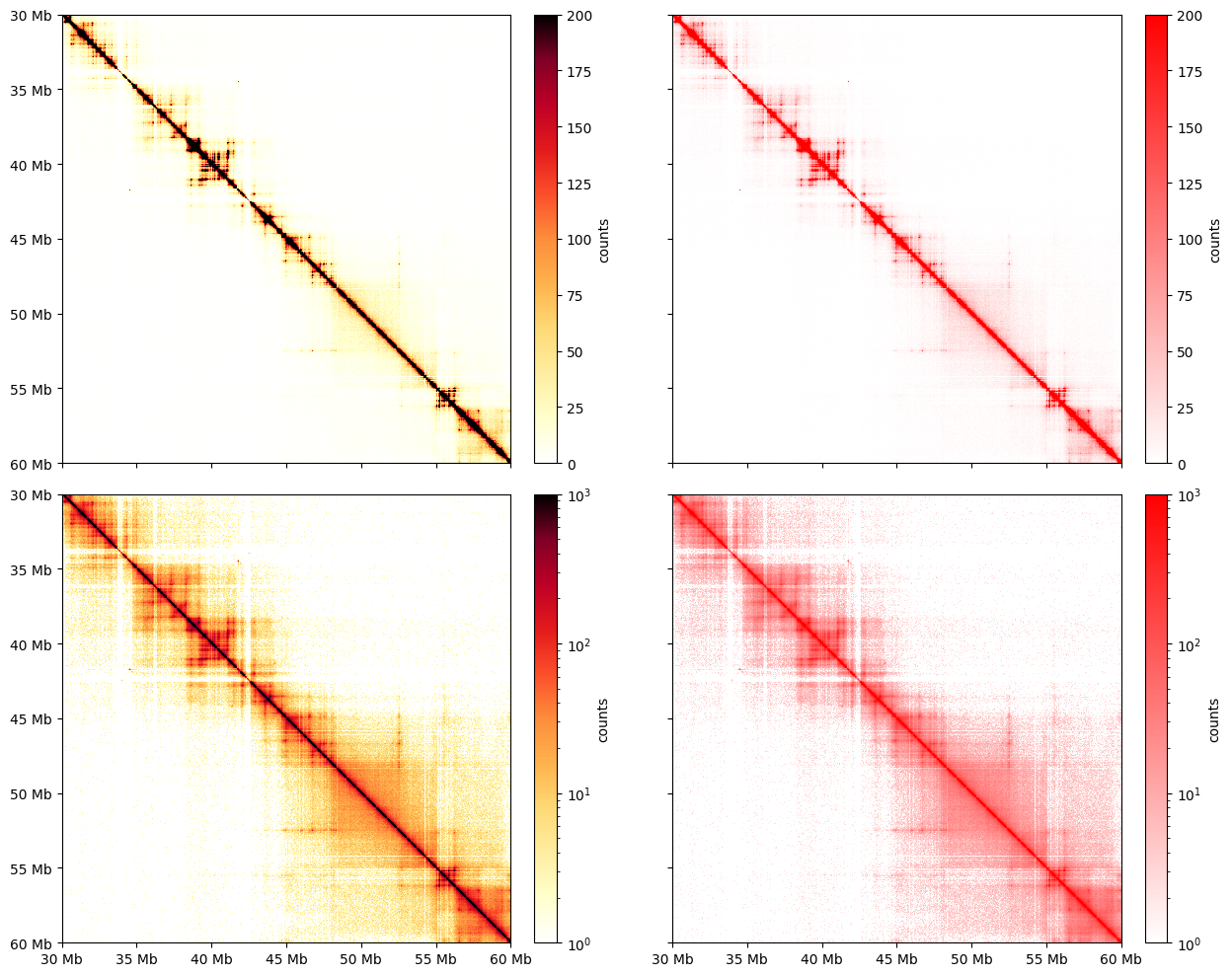
Colormaps
cooltools.lib.plotting registers a set of colormaps that are useful for visualizing C data. In particular, the fall colormap (inspired by colorbrewer) offers a high dynamic range, linear, option for visualizing Hi-C matrices. This often displays features more clearly than red colormaps.
[9]:
### plot the corrected data in fall heatmap and compare to the white-red colormap ###
### thanks for the alternative collormap naming to https://twitter.com/HiC_memes/status/1286326919122825221/photo/1###
import cooltools.lib.plotting
vmax = 5000
norm = LogNorm(vmin=1, vmax=100_000)
fruitpunch = sns.blend_palette(['white', 'red'], as_cmap=True)
f, axs = plt.subplots(
figsize=(13, 10),
nrows=2,
ncols=2,
sharex=True, sharey=True)
ax = axs[0, 0]
ax.set_title('Pumpkin Spice')
im = ax.matshow(clr.matrix(balance=False)[:], vmax=vmax, cmap='fall');
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='counts (linear)');
plt.xticks(chromstarts,clr.chromnames);
ax = axs[0, 1]
ax.set_title('Fruit Punch')
im3 = ax.matshow(clr.matrix(balance=False)[:], vmax=vmax, cmap=fruitpunch);
plt.colorbar(im3, ax=ax, fraction=0.046, pad=0.04, label='counts (linear)');
plt.xticks(chromstarts,clr.chromnames);
ax = axs[1, 0]
im = ax.matshow(clr.matrix(balance=False)[:], norm=norm, cmap='fall');
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='counts (log)');
plt.xticks(chromstarts,clr.chromnames);
ax = axs[1, 1]
im3 = ax.matshow(clr.matrix(balance=False)[:], norm=norm, cmap=fruitpunch);
plt.colorbar(im3, ax=ax, fraction=0.046, pad=0.04, label='counts (log)');
plt.xticks(chromstarts,clr.chromnames);
plt.tight_layout()
The utility of fall colormaps becomes more noticeable at higher resolutions and higher degrees of zoom.
[10]:
### plot the corrected data in fall heatmap ###
import cooltools.lib.plotting
clr_10kb = cooler.Cooler(f'{data_dir}/test.mcool::resolutions/10000')
region = 'chr17:30,000,000-35,000,000'
extents = (start, end, end, start)
norm = LogNorm(vmin=1, vmax=1000)
f, axs = plt.subplots(
figsize=(13, 10),
nrows=2,
ncols=2,
sharex=True,
sharey=True
)
ax = axs[0, 0]
im = ax.matshow(
clr_10kb.matrix(balance=False).fetch(region),
cmap='fall',
vmax=200,
extent=extents
);
plt.colorbar(im, ax=ax ,fraction=0.046, pad=0.04, label='counts');
ax = axs[0, 1]
im2 = ax.matshow(
clr_10kb.matrix(balance=False).fetch(region),
cmap=fruitpunch,
vmax=200,
extent=extents
);
plt.colorbar(im2, ax=ax, fraction=0.046, pad=0.04, label='counts');
ax = axs[1, 0]
im = ax.matshow(
clr_10kb.matrix(balance=False).fetch(region),
cmap='fall',
norm=norm,
extent=extents
);
plt.colorbar(im, ax=ax, fraction=0.046, pad=0.04, label='counts');
ax = axs[1, 1]
im2 = ax.matshow(
clr_10kb.matrix(balance=False).fetch(region),
cmap=fruitpunch,
norm=norm,
extent=extents
);
plt.colorbar(im2, ax=ax, fraction=0.046, pad=0.04, label='counts');
for ax in axs.ravel():
format_ticks(ax, rotate=False)
plt.tight_layout()
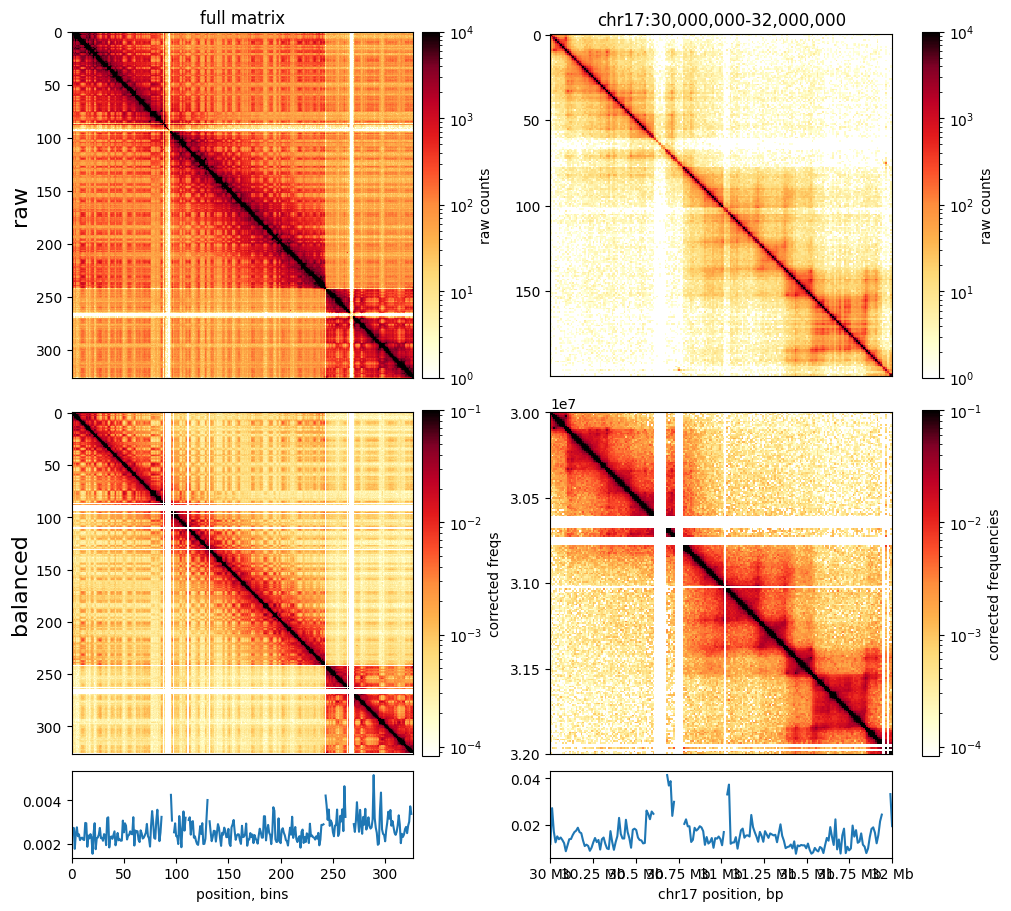
Balancing
When (balance=True) is passed to cooler.matrix(), this applies correction weights calculated from matrix balancing. Matrix balancing (also called iterative correction and KR normalization) removes multiplicative biases, which constitute the majority of known biases, from C data. By default, the rows & columns of the matrix are normalized to sum to one (note that the colormap scale differs after balancing). Biases, also called weights for normalization, are stored in the weight column of
the bin table given by clr.bins().
[11]:
clr.bins()[:3]
[11]:
| chrom | start | end | weight | |
|---|---|---|---|---|
| 0 | chr2 | 0 | 1000000 | 0.002441 |
| 1 | chr2 | 1000000 | 2000000 | 0.002435 |
| 2 | chr2 | 2000000 | 3000000 | 0.002728 |
Before balancing, cooler also applies filters to remove low-coverage bins (note that peri-centromeric bins are completely removed in the normalized data). Filtered bins are stored as np.nan in the weights.
Matrices appear visually smoother after removal of biases. Smoother matrices are expected for chromosomes, as adjacent regions along a chromosome are connected and should only slowly vary in their contact frequencies with other regions.
[12]:
### plot the raw and corrected data in logscale ###
from mpl_toolkits.axes_grid1 import make_axes_locatable
plt_width=4
f, axs = plt.subplots(
figsize=( plt_width+plt_width+2, plt_width+plt_width+1),
ncols=4,
nrows=3,
gridspec_kw={'height_ratios':[4,4,1],"wspace":0.01,'width_ratios':[1,.05,1,.05]},
constrained_layout=True
)
norm = LogNorm(vmax=0.1)
norm_raw = LogNorm(vmin=1, vmax=10_000)
ax = axs[0,0]
im = ax.matshow(
clr.matrix(balance=False)[:],
norm=norm_raw,
cmap='fall',
aspect='auto'
);
ax.xaxis.set_visible(False)
ax.set_title('full matrix')
ax.set_ylabel('raw', fontsize=16)
cax = axs[0,1]
plt.colorbar(im, cax=cax, label='raw counts')
ax = axs[1,0]
im = ax.matshow(
clr.matrix()[:],
norm=norm,
cmap='fall',
);
ax.xaxis.set_visible(False)
ax.set_ylabel('balanced', fontsize=16)
cax = axs[1,1]
plt.colorbar(im, cax=cax, label='corrected freqs')
ax1 = axs[2,0]
weights = clr.bins()[:]['weight'].values
ax1.plot(weights)
ax1.set_xlim([0, len(clr.bins()[:])])
ax1.set_xlabel('position, bins')
ax1 = axs[2,1]
ax1.set_visible(False)
start = 30_000_000
end = 32_000_000
region = ('chr17', start, end)
ax = axs[0,2]
im = ax.matshow(
clr_10kb.matrix(balance=False).fetch(region),
norm=norm_raw,
cmap='fall'
);
ax.set_title(f'chr17:{start:,}-{end:,}')
ax.xaxis.set_visible(False)
cax = axs[0,3]
plt.colorbar(im, cax=cax, label='raw counts');
ax = axs[1,2]
im = ax.matshow(
clr_10kb.matrix().fetch(region),
norm=norm,
cmap='fall',
extent=(start, end, end, start)
);
ax.xaxis.set_visible(False)
cax = axs[1,3]
plt.colorbar(im, cax=cax, label='corrected frequencies');
ax1 = axs[2,2]
weights = clr_10kb.bins().fetch(region)['weight'].values
ax1.plot(
np.linspace(start, end, len(weights)),
weights
)
format_ticks(ax1, y=False, rotate=False)
ax1.set_xlim(start, end);
ax1.set_xlabel('chr17 position, bp')
ax1 = axs[2,3]
ax1.set_visible(False)
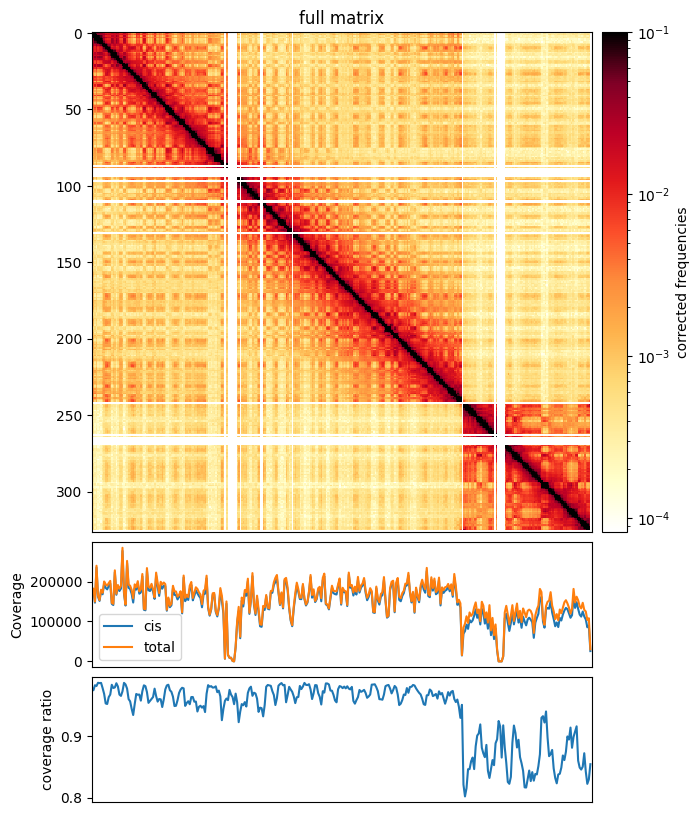
Coverage
Contact matrices often display varible tendency to make contacts within versus between chromosomes. This can be calculated in cooltools with cooltools.coverage and is often plotted as a ratio of (cis_coverage/total_coverage). Note that the total coverge is similar to, but distinct from, the iteratively calculated balancing weights (see above).
[13]:
cis_coverage, tot_coverage = cooltools.coverage(clr)
f, ax = plt.subplots(
figsize=(15, 10),
)
norm = LogNorm(vmax=0.1)
im = ax.matshow(
clr.matrix()[:],
norm=norm,
cmap='fall'
);
divider = make_axes_locatable(ax)
cax = divider.append_axes("right", size="5%", pad=0.1)
plt.colorbar(im, cax=cax, label='corrected frequencies');
ax.set_title('full matrix')
ax.xaxis.set_visible(False)
ax1 = divider.append_axes("bottom", size="25%", pad=0.1, sharex=ax)
weights = clr.bins()[:]['weight'].values
ax1.plot( cis_coverage, label='cis')
ax1.plot( tot_coverage, label='total')
ax1.set_xlim([0, len(clr.bins()[:])])
ax1.set_ylabel('Coverage')
ax1.legend()
ax1.set_xticks([])
ax2 = divider.append_axes("bottom", size="25%", pad=0.1, sharex=ax)
ax2.plot( cis_coverage/ tot_coverage)
ax2.set_xlim([0, len(clr.bins()[:])])
ax2.set_ylabel('coverage ratio')
[13]:
Text(0, 0.5, 'coverage ratio')
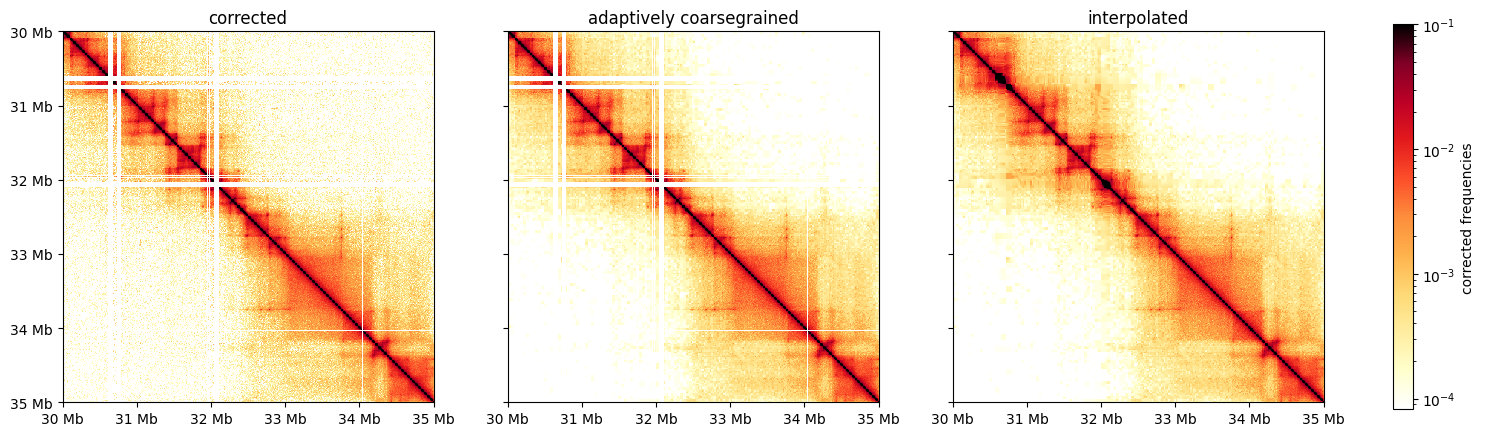
Smoothing & Interpolation
When working with C data at high resolution, it is often useful to smooth matrices. cooltools provides a method, adaptive_coarsegrain, which adaptively smoothing corrected matrices based on the number of counts in raw matrices. For visualization it is also often useful to interpolate over filtered out bins.
[14]:
from cooltools.lib.numutils import adaptive_coarsegrain, interp_nan
clr_10kb = cooler.Cooler(f'{data_dir}/test.mcool::resolutions/10000')
start = 30_000_000
end = 35_000_000
region = ('chr17', start, end)
extents = (start, end, end, start)
cg = adaptive_coarsegrain(clr_10kb.matrix(balance=True).fetch(region),
clr_10kb.matrix(balance=False).fetch(region),
cutoff=3, max_levels=8)
cgi = interp_nan(cg)
f, axs = plt.subplots(
figsize=(18,5),
nrows=1,
ncols=3,
sharex=True, sharey=True)
ax = axs[0]
im = ax.matshow(clr_10kb.matrix(balance=True).fetch(region), cmap='fall', norm=norm, extent=extents)
ax.set_title('corrected')
ax = axs[1]
im2 = ax.matshow(cg, cmap='fall', norm=norm, extent=extents)
ax.set_title(f'adaptively coarsegrained')
ax = axs[2]
im3 = ax.matshow(cgi, cmap='fall', norm=norm, extent=extents)
ax.set_title(f'interpolated')
for ax in axs:
format_ticks(ax, rotate=False)
plt.colorbar(im3, ax=axs, fraction=0.046, label='corrected frequencies')
/home1/rahmanin/.conda/envs/open2c/lib/python3.9/site-packages/cooltools/lib/numutils.py:1376: RuntimeWarning: invalid value encountered in divide
val_cur = ar_cur / armask_cur
[14]:
<matplotlib.colorbar.Colorbar at 0x7f24dcaca730>
[15]:
### future:
# - yeast maps
# - translocations
# - higlass
This page was generated with nbsphinx from /home/docs/checkouts/readthedocs.org/user_builds/cooltools/checkouts/latest/docs/notebooks/viz.ipynb